Catherine Vénien-Bryan, Sorbonne Université IMPMC
-
On 16 June 2023Amphi DEfalse false
-
11h30
Cryo-electron microscopy unveils the gating mechanism of the human Kir2.1 channel
Cryo-electron microscopy unveils the gating mechanism of the human Kir2.1 channel
Catherine Vénien-Bryan, PhD,
Professeur de Biophysique Sorbonne Université
Sorbonne Université IMPMC (Institut de Minéralogie Physique des Matériaux et Cosmochimie) UMR 7590
Abstract
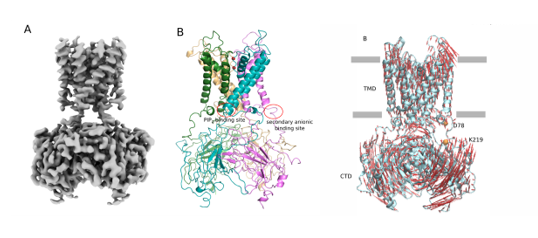
Inward-rectifier potassium (Kir) channels are a group of integral membrane proteins that selectively control the permeation of K+ ions across cell membranes. The small outward K+ current through Kir channels controls the resting membrane potential and membrane excitability, regulates cardiac and neuronal electrical activities, couples insulin secretion to blood glucose levels, and maintains electrolyte balance1. All Kir channels are tetramers and share characteristic structural features. They have a canonical pore-forming transmembrane domain (TMD) made of two transmembrane helices separated by a K+ ion selectivity filter and a large cytoplasmic domain (CTD) containing both N and C termini. The CTD extends the ion conduction pathway and provides docking sites for regulatory ions, proteins, and ligands2. The strong inward-rectification mechanism results from a block on the cytoplasmic side of the channels by endogenous polyamines and Mg2+ that plug the channel pore at depolarized potentials. In addition to being inwardly rectifying, the gating of Kir2.1 channels are selectively activated by the lipid phosphatidylinositol 4,5-bisphosphate (PIP2). The physiological importance of these channels is underpinned by the fact that mutations in these proteins cause a wide range of pathologies. In order to understand the gating mechanism, both structures of Kir2.1 in the closed and open state are needed.
We used cryo–electron microscopy (cryo-EM) combined with image analysis to elucidate the structure of a human Kir channel, Kir2.1 in the closed state3. Furthermore, computational investigations reveal crucial conformational movements, including compaction of the structure and opening movements at the interface between the TMD and CTD, which could facilitate the binding of PIP2. In addition, I will present the cryo-EM structure of the human Kir2.1 channel complexed tp PIP2 in the open state that we have recently solved at 2.9Å resolution. Comparative structural analysis of the Kir2.1/PIP2 complex (open state) with apo-Kir2.1 (closed state) reveals the structural changes that lead to channel gating. I will also present the effect of two mutations on the gating mechanism of this channel. These data will help to understand the pathological mechanisms associated with mutations in the human Kir2.1 channel.
References
1. Hibino et al. Physiol. Rev. 90 291–366 (2010).
2. Fürst et al. Front. Physiol. 4, 404 (2014).
3. Fernandes, Zuniga, et al. Sci. Adv., 8, eabq8489 (2022)
Biography






